Commercializing First-in-Class EndoAVF Technology from Seed to Acquisition
Client Snapshot
Industry: Startup
Stage: Seed to Exit
Project Type: Full Commercialization & Medical Affairs Strategy
The Challenge
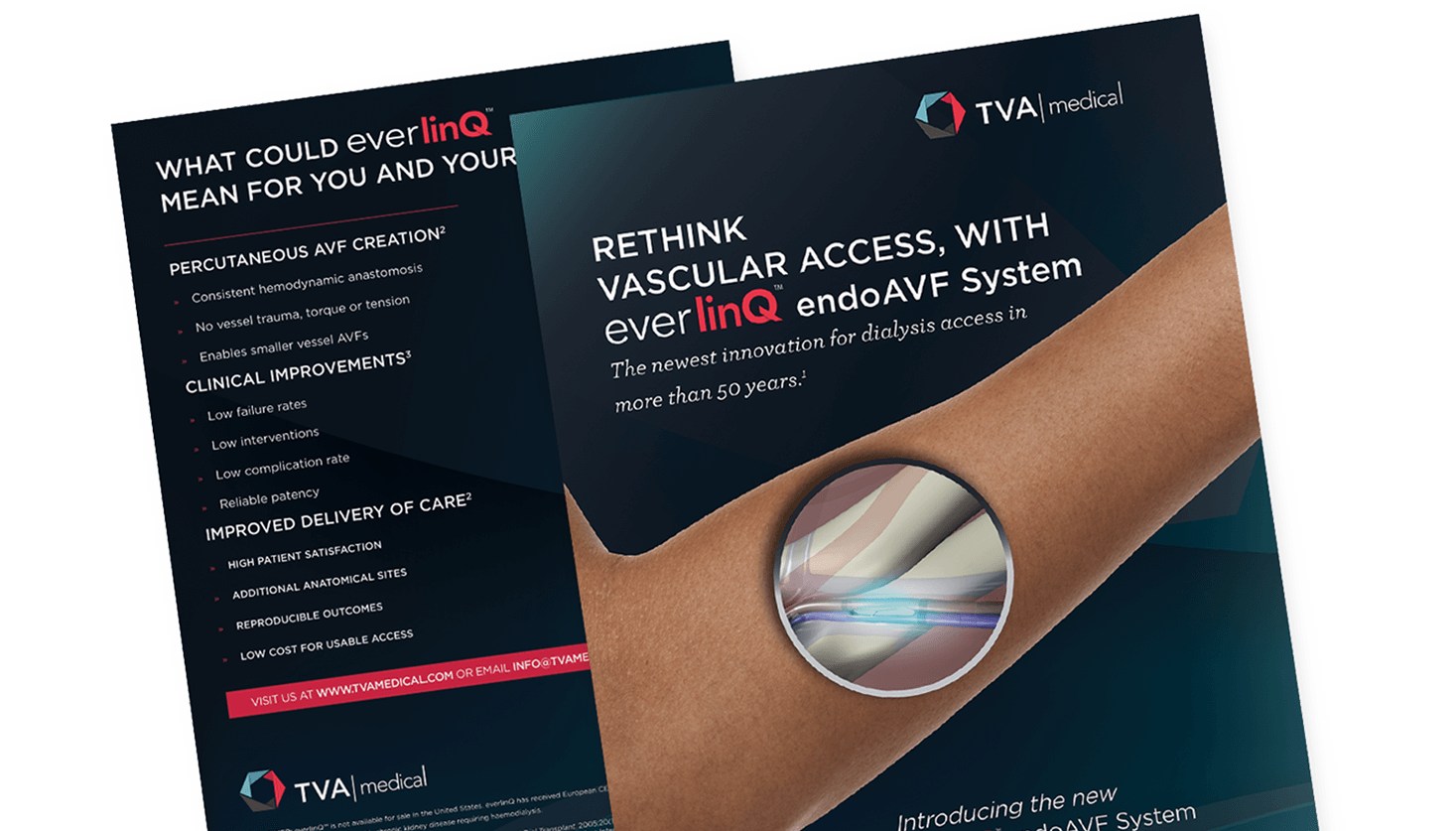
BD (formerly TVA Medical), a startup medical device company, developed the first minimally invasive endovascular solution for arteriovenous fistula (AVF) creation in dialysis patients. With pivotal trials complete and CE Mark secured, the company needed to generate market momentum, align clinical and commercial strategies, and build confidence with investors and clinicians to position the organization for acquisition. Physician resistance, reimbursement hurdles, and the need for a unified brand platform posed significant challenges.
Our Approach

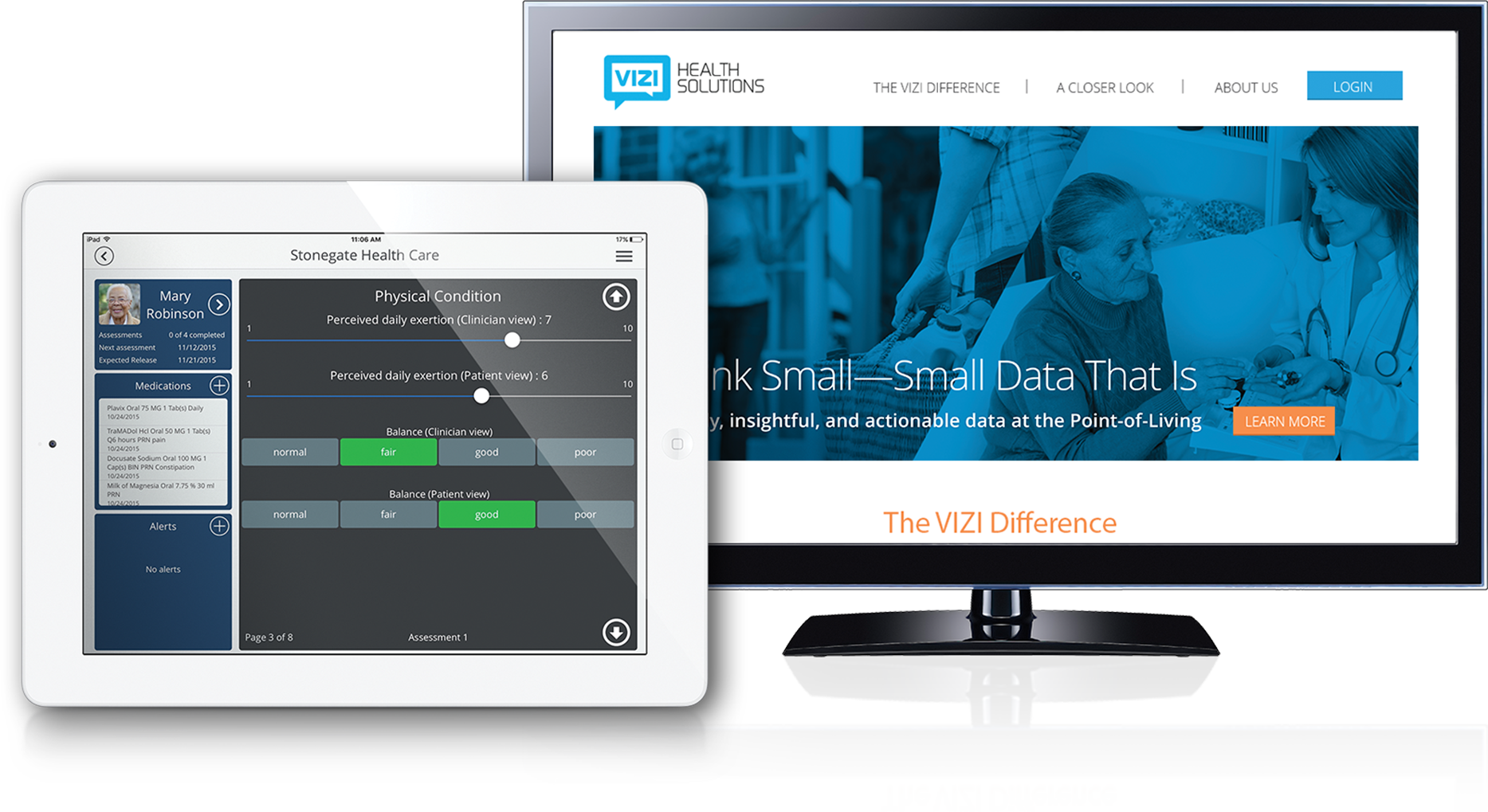
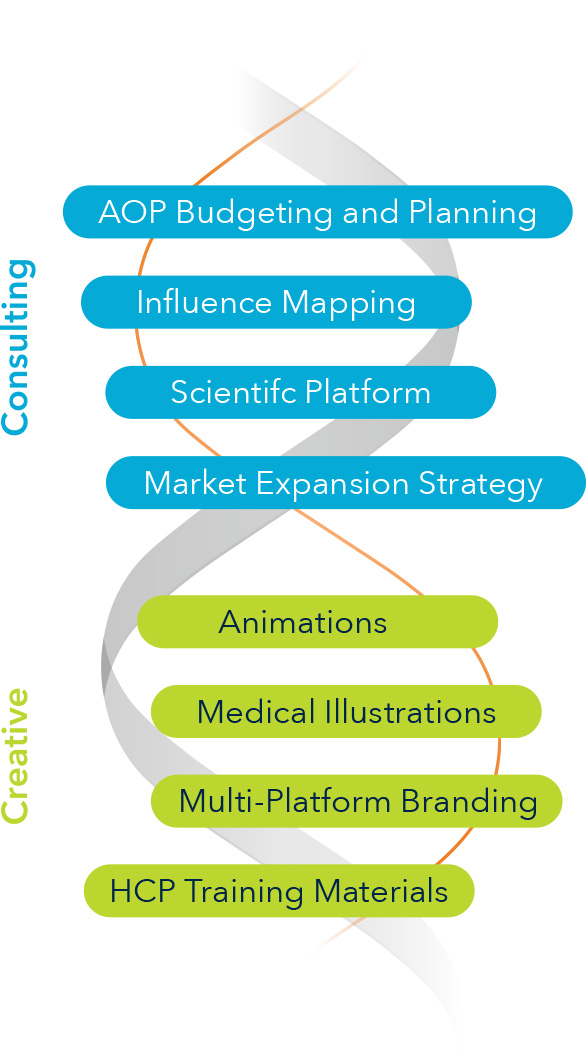
LLSS partnered with TVA Medical—now part of BD—from early funding through acquisition. We developed and executed the global commercialization plan, combining clinical education, medical affairs strategy, and creative execution. Our team co-created materials with KOLs, medical affairs, and commercial stakeholders to support publication planning, advocacy building, patient education, and procedure adoption. Following acquisition by BD, LLSS supported rebranding efforts for what is now known as the WavelinQ™ EndoAVF System.
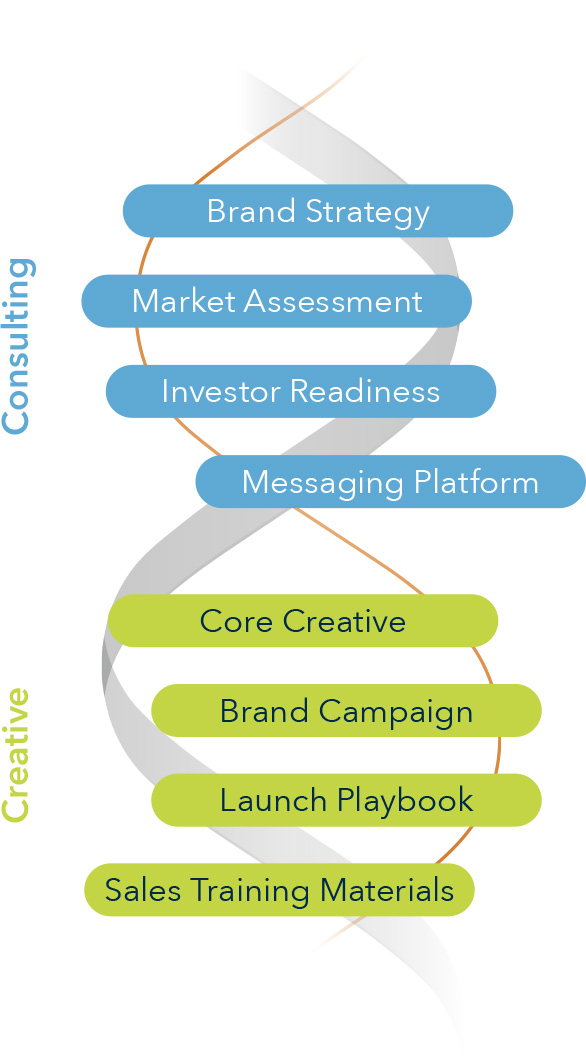
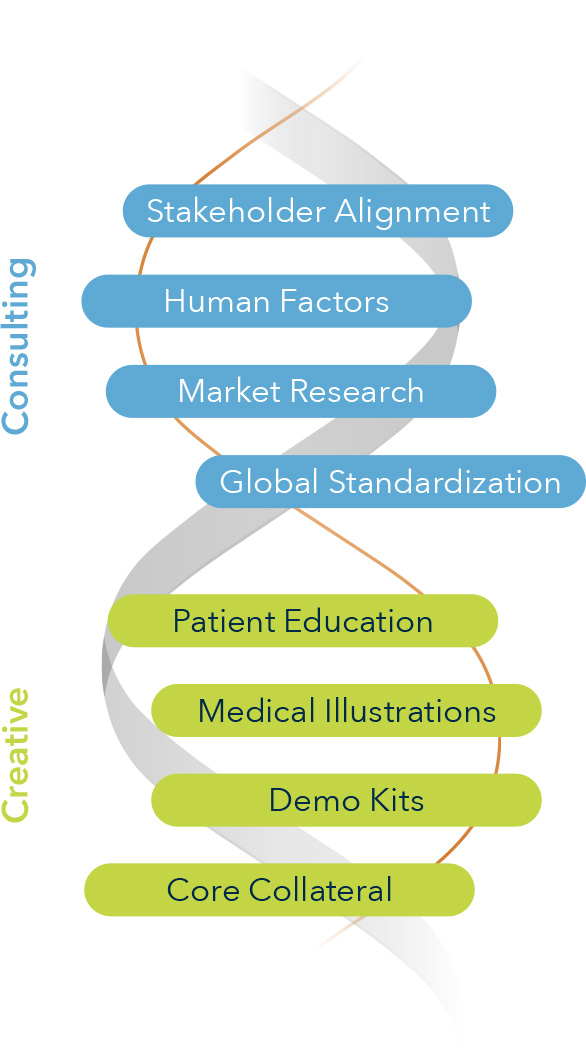
Solutions Delivered
- Brand identity and scientific platform development
- Medical affairs and publication planning strategy
- Sales training materials and demo support
- Clinical trial and claims matrix planning
- Advisory boards and KOL engagement
- Patient and provider education tools
- Tactical assets including animations, brochures, booth graphics, and onboarding kits
- Post-acquisition brand transition and launch support
Outcomes & Results
- Secured multiple rounds of funding including Series C
- Established a comprehensive clinical communication and medical education strategy
- Created foundational materials that enabled clinical and commercial alignment
- Successfully positioned the company for acquisitionby BD
- Product rebranded and relaunched as WavelinQ™ EndoAVF, now a standard of care in ESKD